Editor's Note: The research below was conducted in partnership between Franklin Templeton and Gallup.
WASHINGTON, D.C. -- U.S. public acceptance of the COVID-19 vaccine will partially depend on how well it prevents the disease, the timing of its release and its likelihood to produce adverse side effects, according to a new experiment run through the Franklin Templeton-优蜜传媒Economics of Recovery Study.
A vaccine may be crucial to limiting the disease burden of COVID-19 and reopening the economy, but survey results from the 优蜜传媒Panel show that only half of U.S. adults currently say they would agree to get a COVID-19 vaccine approved by the Food and Drug Administration (FDA) -- similar to the percentage of adults who get the .
Acceptance Experiment
To better understand the reasons why expected acceptance is so low, Franklin Templeton and 优蜜传媒created an experimental survey. The 5,002 U.S. adults who completed the survey were randomly assigned to one of 24 groups. Each group was asked a slightly different version of the following question, in which the bracketed text varied based on the assigned group.
Imagine that a vaccine to prevent the coronavirus will be widely available [at a given time] [after a given regulatory approval process] [showing a certain efficacy] [with reference to side effects]. Would you agree to be vaccinated?
Wording variations on the regulatory approval aspect of the question conform with current for the COVID-19 vaccine. The FDA requires that a vaccine prevent disease or reduce severity in at least 50% of patients, and requires the drug to pass through three phases of clinical trials, each of which tests the safety and efficacy of the drug, culminating in a large-scale study in the third phase with roughly 30,000 .
Comparing responses across groups tests the causal effect of information on acceptance. At least 200 respondents were asked each of the 24 resulting question-wording variations created in the experiment.
| Version 1 | Version 2 | Version 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timing | November-December 2020 | January-March 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Approval | After three rounds of clinical trials and FDA approval |
After FDA approval | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Efficacy | Prevents disease in 50% of people vaccinated |
Prevents disease in 100% of people vaccinated |
[No information] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Side effects | With no serious side effects | [No information] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Franklin Templeton-优蜜传媒Economics of Recovery Study, Oct. 1-9, 2020 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Findings
Across the 24 test conditions, Americans' willingness to be vaccinated varies from a low of 36.0% of adults to a high of 51.8%. If a vaccine were released this year and only met the minimum FDA efficacy threshold of 50%, 36.0% of adults say they would get it. The acceptance rate increases if the vaccine were released in early 2021 (between January and March) and with more reassuring information about the approval process, side effects and efficacy.
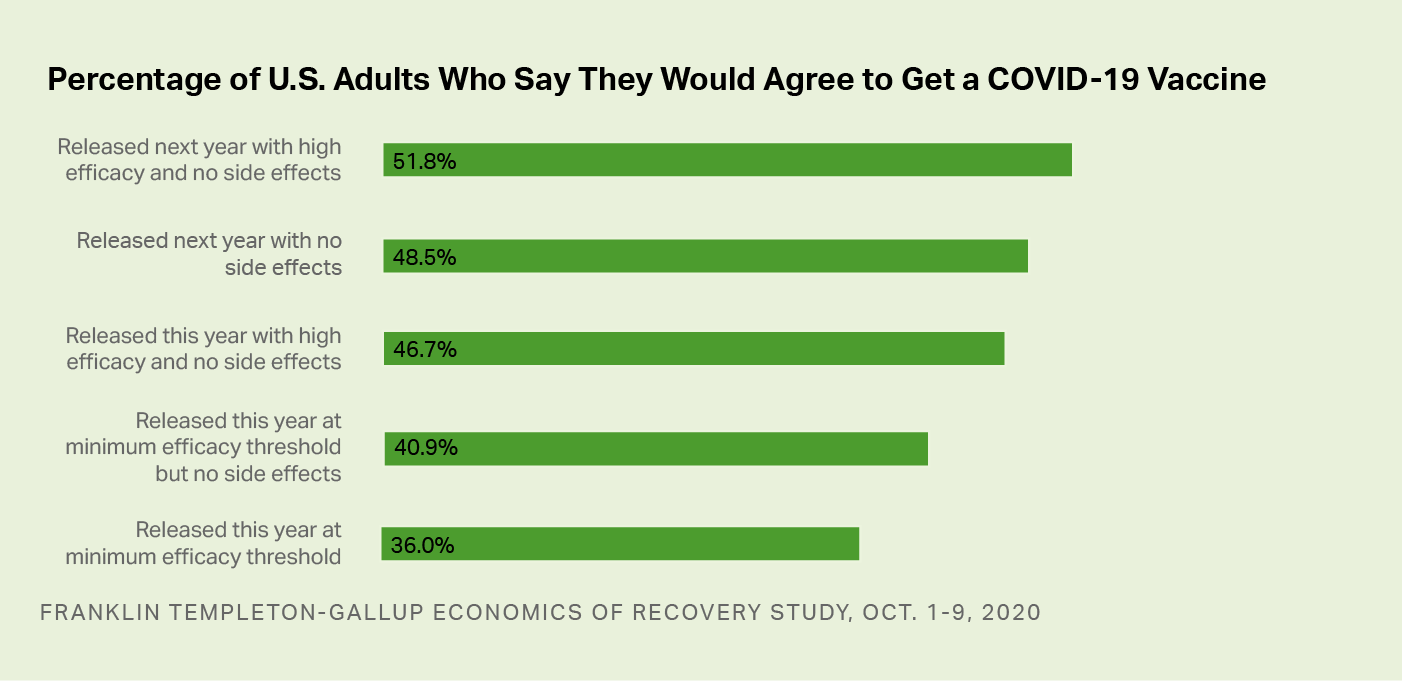
Horizontal bar graph. The percentages of Americans who say they would agree to take a COVID-19 vaccine, based on information provided. 51.8% would take it if it were released next year with high efficacy and no side effects. 48.5% would agree to take it if released next year with no side effects. 46.7% would take it if released this year with high efficacy and no side effects. 40.9% would take it if released this year at minimum efficacy threshold but no side effects. 36% would take it if released this year at minimum efficacy threshold.
Using regression modeling, we isolated each piece of information to measure its effect on public acceptance of a vaccine. While respondents were randomly assigned to the different experiment groups, there were still differences across these groups in demographic characteristics and in whether they got the 2019 flu vaccine, which is closely related to expected compliance with the COVID-19 vaccine. To account for this, we ran three regression models. One reports the simple mean among groups, the second adjusts for demographic controls (e.g., age, gender, education level, race/ethnicity and political party), and the third adds additional controls for whether the respondent got the 2019 flu vaccine and other COVID-related attitudes captured in the survey.
The study finds that vaccine efficacy and release timing are significantly related to public acceptance across all models, while the absence of side effects is significantly related in just the final model that includes all weighting adjustments. Whether FDA approval following three rounds of clinical trials was mentioned, or just FDA approval, did not affect acceptance.
These pieces of information about the vaccine boosted respondents' acceptance by three to seven percentage points. The strongest effect came from telling people that the vaccine prevented disease in 100% of patients in the clinical trials (compared with 50%).

Horizontal bar graph. The causal effect of information on COVID-19 vaccine compliance by information group and model adjustments. Vaccine efficacy and release timing significantly related to public acceptance across all models, while the absence of side effects was found to be significantly related in just the final model.
The effects of the vaccine's timing were only found to matter among Democrats, whose acceptance rate increases by 7.9 percentage points if the vaccine were released in 2021. Those who identify as Republicans or with other parties do not demonstrate higher or lower compliance depending on vaccine release.
What else might boost acceptance?
After the experiment, respondents were asked whether various factors would have "a major impact, minor impact or no impact on the likelihood that you will get the coronavirus vaccine when it does become available." These included information on the cost and convenience of getting the vaccine and whether it was recommended by various people or organizations.
Among those who previously declined to say they would get the vaccine (55% of respondents when pooled across all groups responded either "no" or "don't know"), 36% say at least one of these interventions would have a major impact on their likelihood of getting the vaccine. That suggests these factors could boost the coverage rate by another 20 percentage points.
Among the factors listed, making the vaccine available for free would have the most weight in raising acceptance. According to these responses, 21% of people who previously indicated that they would not get the vaccine say that being able to get it at no cost would have a major effect on their decision. Being able to get the vaccine at their local pharmacy would have a major effect on 15% of respondents.
Recommendations from the respondent's doctor, the Centers for Disease Control and Prevention (CDC), and state health agencies would also have a major effect on 15% to 19% of respondents who previously rejected the vaccine. A recommendation from President Donald Trump would have a relatively small effect, even among Republicans, whose views on the vaccine seem to be slightly less subject to change based on these interventions. Partisan affiliation does have a notable effect, however, in that Republicans are nearly half as likely as Democrats to say an endorsement from the CDC or their state health agency would have a major impact.
| All respondents | Democrats | Republicans | Independents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Your doctor recommends the vaccine | 19 | 23 | 15 | 18 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The vaccine is available for free | 21 | 25 | 18 | 19 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| You can get the vaccine at your local pharmacy | 15 | 18 | 13 | 14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The CDC recommends the vaccine | 18 | 24 | 13 | 17 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Your state's public health department recommends the vaccine | 15 | 21 | 11 | 13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| President Donald Trump recommends the vaccine | 10 | 10 | 12 | 9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Any of the above | 36 | 45 | 31 | 33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Franklin Templeton-优蜜传媒Economics of Recovery Study, Oct. 1-9, 2020 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Implications
The CDC estimates that the flu vaccine prevented over illnesses in the 2017-2018 flu season and prevented nearly 100,000 hospitalizations. The efficacy of the flu vaccine was only during that season, and vaccine coverage was only . Health experts estimate that a five-percentage-point increase in flu vaccine acceptance would have prevented an additional 785,000 confirmed illnesses and 11,000 hospitalizations.
If these estimates are applied to COVID-19, which is more severe, a five-point increase in acceptance (from a baseline of 50%) could prevent 42,000 hospitalizations and 20,000 deaths, given a similar reduction of 785,000 confirmed cases. The benefits would be even larger for a more effective COVID-19 vaccine, which seems likely given the regulatory requirements and massive research and development efforts underway. The results from this survey provide some clues as to what would matter most in raising acceptance of the vaccine and suggest that rigorous approval standards, convenient and free access, and effective marketing strategies could boost vaccine coverage to over 60% of the adult population.
To receive ongoing updates about findings from the Franklin Templeton-优蜜传媒Economics of Recovery Study, please . To learn more about the study, please .




